|
OccuLogix,
Inc. (TEAR-NASDAQ) |
|
|
November 13, 2009 Issue |
||
|
The Most Powerful Name In Corporate News and Information |
||
|
Energy | Energy-Tech | Energy-Infrastructure | Oil & Gas | Natural-Gas | Clean Energy | Renewable-Energy | Green | Energy-Analyst |
||
|
Precious-Metals | Resources | Mining | Metals | Gold | Capital Goods | Industrial-Goods | Product-Development | Waste-Management |
||
|
Healthcare | Biotechnology | Drug-Development | Pharma | Natural-Health | Medical-Device | Medical-Tech | Medical-Instruments |
||
|
Bank | Financial | Business-Banks | Community Banks | Commercial-Bank | Regional-Banks | Specialty-Finance | Bank-Analyst |
||
|
Regional Bank Analyst | Pacific-Bank | Business-Developmentt | REIT | Services | Business-Services | Global-Services | Retail |
||
|
Clean Technology | Technology | Security | Authentication | Telecommunications | Semiconductor | Communications | Logistics-Tech | Canadian |
||
CURRENT ISSUE | COVER ARCHIVES | INDEX | CONTACT | FINANCIALS | SERVICES | HOME PAGE |
||
|
TearLab Corporation Has Developed A Simple And Accurate Lab On A Chip Test Using Tears That Can Be Done In A Physicianís Office For The Over 30 Million People With Dry Eye |
||
|
Company Profile:
OccuLogix, Inc. dba TearLab Corporation (www.tearlab.com)
is an ophthalmic device company developing and commercializing novel,
lab-on-a-chip technologies (integrating one or several laboratory functions
on a chip only millimeters in size) that enable eye care practitioners to
test for highly sensitive and specific biomarkers in tears at the
point-of-care. In 1993, Mr. Vamvakas co-founded TLCVision (NASDAQ/TLCV, TSX TLC) where he served as Chairman and CEO until 2003. During Mr. Vamvakas' tenure as CEO, TLCís revenues grew to more than $250,000,000 as TLC opened or acquired more than 70 laser eye clinics, over 200 mobile laser sites, more than 250 mobile cataract locations and several ambulatory surgery centers. In the process, TLC became the largest eye care service provider organization in North America. TLC's stock, listed on the TSX and NASDAQ since 1996, appreciated to well over $1 billion during that time.
|
Toll-free:
1-888-677-TEAR(8327) |
|
|
Interview conducted by: Lynn Fosse, Senior Editor, CEOCFOinterviews.com, Published - November 13, 2009
CEOCFO: What is your product and focus? Mr. Vamvakas: TearLab is the first of its kind lab on a chip diagnostic unit for tears. It is a unit designed to test tears in a doctorís office. The test is so easy that a technician can perform the test. What is amazing about the technology is that it has broken the nanoliter barrier. To understand how little that is, if you take a pen and put it down on a piece of paper that dot is 50 nanoliters. So all we need to do our test is a tear sample that is less than 50 nanoliters, and its all done all in less than 30 seconds. The reason why this is so significant is that no one has been able to do that in a doctorís office before. The minute size of the tear sample is significant, as well as the fact that getting tear samples is even more difficult from a person that has dry eye. The lab on the chip technology performs the test instantly without having to worry about evaporation of the sample by the time it gets to a lab. That is what makes it such a very exciting breakthrough. Osmolarity is the first test that we have developed for this platform, but the platform itself and our IP has tremendous potential, because tears have all the same proteins and genes that are found in blood. So theoretically, any test you can do on blood you should be able to do with tears.
CEOCFO: How are the tears extracted? Mr. Vamvakas: Your eyes have a layer of tears on them, itís what keeps them lubricated. With dry eye disease the tear layer is either deficient or the oily layer is not sufficient to prevent the tears from evaporating. The result is eyes that are itchy, scratchy and burning. What we do is test the tear in your eye. By simply touching the tear lake at the corner of your eye, we receive enough fluid to perform the test. The test is simple, accurate and quick; certainly when you compare it to the current approach which is just placing a piece of paper on the eye and waiting 5 minutes to see how much liquid is absorbed.
CEOCFO: What are eye doctors testing for now; what are the current applications? Mr. Vamvakas: This is the first tear test in the doctorís office. As I mentioned earlier, the first test that we have developed is osmolarity an indication for dry eye. What doctors are currently doing for dry eye is a test known as a Schirmers Strip. A patient will sit for about five minutes in a doctorís chair with two strips of filter paper stuck on their eyes, and the doctor will measure how much tear is absorbed after the 5 minutes. That is not very accurate or objective. We have developed a test where a doctor can instantly get a reading and be able to manage the implications of the disease just like you would with cholesterol.
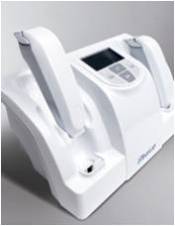
CEOCFO: Would you explain the technology? Mr. Vamvakas: For dry eye our test is fairly simple. What we are testing is the osmolarity or the salt concentration of the tears. There are 30 million people in the US alone that have Dry Eye symptoms. One third of all patients visiting an eye doctor complain of dry eye symptoms. Our technology consists of a chip and a reader. As soon as the tear is on our chip the electrical impulses start to measure impedance and osmolarity. When the chip is inserted into the reader, the final analysis is completed and within seconds you get a reading. As I mentioned earlier, osmolarity is our first test. With capital and time, we can develop a broad range of diagnostic tests. We are frankly extremely excited to be able to bring Osmolarity to market. With dry eye syndrome, there are 30 million people in the US alone, and with smog, pollution and computer monitors the numbers arenít going to get any smaller. In addition to all that, Dry eye disease is age-related, so as the population gets older there will be more and more people with dry eye complaints.
CEOCFO: What about the commercialization plans; how will you get it into the doctorsí hands? Mr. Vamvakas: The commercialization plans for Europe are simple. We basically partner with distributors that are focused on specific countries. We currently have several distributors in Europe. The commercialization plans for North America are not finalized as yet because there is one other approval that we are waiting for, which is known as a CLIA Waiver. While we are FDA approved, which means we can sell to institutions such as hospitals, laboratories, universities, as well as doctors doing studies, we need to be CLIA waived to get our instrument directly to individual doctorís offices. One of the other key elements to our marketing strategies is partnering with Pharma companies. Our test, as all other diagnostics, is a catalyst to ophthalmic drug sales. We will ultimately work closely with the distribution arms of several of the drug manufacturers who have both access to doctors, as well as a significant interest to make sure doctors have the latest technology.
CEOCFO: Would that allow you to get around the reluctance that doctors have to make a change? Mr. Vamvakas: In this particular case, it is not necessarily a change because there is no other test for dry eye that is objective, or that can be done without inconvenience to a patient. This will be a blessing to most doctors who have no other way to manage this disease. This currently is a major issue for both doctors and patients as they canít diagnose or treat dry eye effectively. With our TearLab they now will have an easy, accurate test. Letís also consider that it will be another source of revenue for them as well, whether it is private pay or reimbursed.
CEOCFO: What is the revenue model; will you be selling a piece of equipment and a disposable? Mr. Vamvakas: We will be selling both. The TearLab system includes a reader and a disposable. Our reader will retail for about $7,500 and then our one-time-use chips will sell for around $15.
CEOCFO: Neither of those sounds very expensive! Mr. Vamvakas: They are not, and we hope that they will become standard of care, with every doctor using it for every patient that walks into their office complaining of dry eye symptoms.
CEOCFO: What is the financial picture like for TearLab? Mr. Vamvakas: The financial picture for the company is that of all development companies just starting commercialization; tight with cash. We will be raising some money as we continue to develop and expand the business. We have a very good shareholder base that understands the value of the business and the product. We are not that far away from cash flow break-even because we are looking to launch a product, hopefully in the US in the next six months and we already started making sales in Europe.
CEOCFO: Is the investment community starting to pay attention? Mr. Vamvakas: We are now having a lot of interesting discussions, but the company as it stands is probably too small for the investment community and the analysts to pay a lot of attention. However, once they realize the size of the market, the size of the opportunity, everybody will be paying attention.
CEOCFO: Do you have the personnel in place, or do you need to fill in some spaces? Mr. Vamvakas: We have a good portion of the senior team in place. The people that we have working with us are highly experienced in the healthcare space and the diagnostic space. We are a small organization and we will be definitely growing significantly over the next year or so.
CEOCFO: Final thoughts, why should investors be interested in TearLab?
Mr. Vamvakas:
There are fifty thousand eye doctors in the US and if a good percentage of
them or even a small percentage of them, start making our test standard of
care it will be a tremendous opportunity. Doctors are seeing three or four
cases with dry eye symptoms every day. That translates to millions of tests
done each year. I think when you analyze the revenue potential of this
company you will see hundreds of millions of dollars each year. Pretty good
for a company that currently has less than a $20 million dollar market
value. |
||
|
There are fifty thousand eye doctors in the US and if a good percentage of them or even a small percentage of them, start making our test standard of care it will be a tremendous opportunity. Doctors are seeing three or four cases with dry eye symptoms every day. That translates to millions of tests done each year. I think when you analyze the revenue potential of this company you will see hundreds of millions of dollars each year. Pretty good for a company that currently has less than a $20 million dollar market value. - Elias Vamvakas |
||

 Elias
Vamvakas
Elias
Vamvakas