Precigen poised to disrupt gene and cell therapy industry with UltraCAR-T therapeutic platform
 Dr. Helen Sabzevari
Dr. Helen Sabzevari
President & CEO
Precigen, Inc.
(Nasdaq: PGEN)
www.precigen.com
Interview conducted by:
Lynn Fosse, Senior Editor, CEOCFO Magazine
Published – April 27, 2020
Company Contact:
Rutul Shah
(301) 556-9829
rshah@precigen.com
Investor Relations Contact:
Steven Harasym
(301) 556-9850
sharasym@precigen.com
CEOCFO: Dr. Sabzevari, according to the Precigen website, you are “Advancing Medicine with Precision.” What does that mean for Precigen?
Dr. Sabzevari: Advancing medicine with precision means that we are focusing on getting the right medicine to the right patient at the right time rather than taking a one-size-fits-all approach. Each patient is unique and the way to target a disease for each patient may be different based on numerous factors. Precigen’s core therapeutic focus areas include immuno-oncology, infectious diseases and autoimmune disorders; however, I think the concept of precision medicine is easiest to understand in oncology where over the past decades, non-surgical treatments were largely limited to radiation and chemotherapy. Those treatments are generic, non-targeted and applied to all patients without really taking into account the specific characteristics of the disease or the patient population. While these treatment options can be very effective for certain types of cancer, they aren’t effective for all patients and sometimes the side effects can be as debilitating as the disease.
At Precigen, precision medicine is about identifying a therapeutic target in a specific indication and then designing our therapies to target patient populations that will stand to benefit most from that treatment.
CEOCFO: Would you give us some examples of what you are working on and how the precision part comes into play?
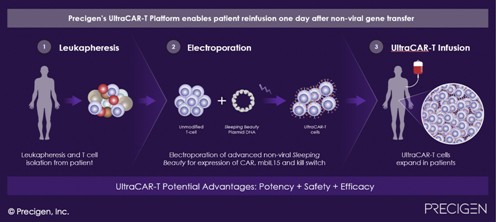
Dr. Sabzevari: In the field of immuno-oncology, a fairly new field of research focused on activating the body’s immune system to fight cancer, Precigen has designed a unique and differentiated therapeutic platform called UltraCAR-T™. UltraCAR-T is designed to super charge a person’s T cells to attack a precise tumor target. T cells are part of the immune system and protect the body from pathogens and cancer cells.
With UltraCAR-T, after a blood draw at a hospital, the patient’s own T cells are genetically programmed to recognize an antigen on a patient’s tumor cells and kill them. With first generation CAR-T therapies, there is a long, complex and expensive manufacturing process that happens at an offsite manufacturing facility and can take up to 3 to 4 weeks. With UltraCAR-T, we have bypassed the need for this type of manufacturing and have created a rapid manufacturing process that is done at the hospital. This means that we can manufacture UltraCAR-T overnight, reinfusing the patient with their own reprogrammed T cells that are now armed and tailored to battle a specific tumor type. This is one of our most advanced and exciting therapeutic platforms and we believe we are the only company successfully doing this right now.
We now have two UltraCAR-T programs in clinical trials. One program, PRGN-3005, is for treatment of ovarian cancer, a solid tumor, conducted in collaboration with the University of Washington and Fred Hutchinson Cancer Research Center. The ovarian cancer patient population has very few treatment choices and the rate of success has been very low.
Our second UltraCAR-T program, PRGN-3006, is in a type of hematological cancer called acute myeloid leukemia (AML) and is conducted in collaboration with the Moffitt Cancer Center. AML is an orphan disease with very poor prognosis. Five‐year survival rates are approximately 25 percent overall across all age ranges and for elderly AML patients over 65 years of age, median survival is shorter, ranging from 3.5 months to 1.4 months, depending on a patient’s age. Time is of the essence for these patients and they cannot wait for lengthy manufacturing processes, which is why UltraCAR-T could be a real game-changer for these patients. As I explained with UltraCAR-T, we can manufacture and reinfuse the patient the next day.
CEOCFO: How have you decided what conditions to consider? Are some easier than others?
Dr. Sabzevari: We take a science-driven approach to drug development and look for diseases where there are limited or no treatment options or where current treatment options fall short for patients. When we decide to pursue a specific indication, there are a number of factors we consider. In addition to patient need, we also factor in the prerequisite technologies needed to develop a therapeutic with the highest potential impact. In our case, we have carefully built a set of complementary technology platforms that allow us to quickly design and deliver gene and cell therapies to target specific diseases.
For example, we have a library of specific vectors that can deliver genetic payloads for expression of multiple therapeutic genes simultaneously. Secondly, we have technology platforms that can deliver these vectors in the most efficient way, both virally and non-virally. Finally and importantly, we have mechanisms to control gene expression through various switches that allow us to turn on and off genes.
We look at the totality of these platforms combined with the diseases we are targeting in patients with high need to carefully design therapeutics where there is science and preclinical evidence suggesting potential for success.
We are also trying to bring cutting-edge treatments to patients at a lower cost. This is very important because currently, for instance, when you look at first generation CAR-T therapies, the price tag can be up to half a million dollars for one treatment regimen. The price alone can be a deciding factor in whether or not the patient receives that therapy and we want physicians to be able to focus on finding therapeutic options that actually work for their patients without fretting over the price.
CEOCFO: What would a hospital need to have in place in order to create this overnight? Equipment, technology, training?
Dr. Sabzevari: From the perspective of the hospital, if they have a “clean room,” which the majority of cancer centers have, then they have what they need to implement UltraCAR-T.
The Precigen team works very closely with the hospital to do a “technology transfer.” We train the technicians at the hospital on how to manufacture UltraCAR-T onsite in their clean room. We have been very successful at transferring the technology needed to manufacture UltraCAR-T for the clinical studies. At Fred Hutchinson Cancer Research Center and at the Moffitt Cancer Center, in ovarian cancer and AML, respectively, we trained their staff and both have been able to successfully manufacture UltraCAR-T overnight and treat patients the next day.
CEOCFO: Would you tell us about the FDA clearance for PRGN-3005 and PRGN-3006?
Dr. Sabzevari: As with any new therapy, the FDA looked at all aspects of the proposed clinical study protocol to ensure patient safety before the study was granted clearance to proceed. For the UltraCAR-T trials, after a comprehensive review of our data packages, which included preclinical data and details regarding our manufacturing process and platform technology, the FDA granted clearance for study initiation.
CEOCFO: Would you tell us about the change of name to Precigen?
Dr. Sabzevari: I originally started in mid-2017 with Intrexon, which had a broad portfolio of commercial areas, one of which was healthcare under the name Precigen. The concept and the vision at Intrexon was to apply DNA technology to various industries, such as health, agriculture, and energy. During the last two years, my team at Precigen focused on building a robust portfolio of preclinical and clinical therapeutic assets that focus on immuno-oncology, autoimmune disorders and infectious diseases. As a result of these efforts, Precigen’s healthcare portfolio became such a potential differentiator in the market and for the treatment of patients that Intrexon’s board and management team determined it would be most valuable to shareholders for the company to pivot to a healthcare-focused organization.
In that context, the decision was made to change the name from Intrexon. As part of this change, we prioritized programs and also parted with certain assets and subsidiaries that did not fit within the vision for the new healthcare-focused company. This has allowed us to generate a cash runway to further progress our healthcare programs.
CEOCFO: What has been the response from the medical and scientific communities? Do people understand what you are doing? Are they somewhat skeptical?
Dr. Sabzevari: I think the scientific community is becoming more and more excited about Precigen as they learn about us and as we begin to progress in the clinic and present preclinical data at medical meetings. For the past two years, we have kept ourselves fairly under the radar. The reason for that was to give us time to develop our platforms, ensuring that they were well-tested and ready for expansion into clinical trials and beyond.
Our UltraCAR-T studies are led by some of the best investigators in the world and they are quite excited about this therapeutic platform because we are offering something that is really different. If these studies are successful, it could disrupt the field of CAR-T therapeutics. That’s an exciting prospect.
CEOCFO: What about the investment community? Do they recognize what you have developed?
Dr. Sabzevari: Our story has been resonating with the investment community in the conversations we are having, especially with the changes that we have made, including a tighter focus on healthcare, a refocusing of resources on priority programs, and driving fiscal responsibility across everything we do.
As Intrexon, we were focusing on everything from apples to salmon to mosquitoes to energy, and of course, health. With all of these activities, it was difficult for analysts and investors, especially in the biotechnology space, to appreciate the value of the healthcare portfolio. As the new Precigen, the investment community is starting to understand the potential value of the health portfolio.
CEOCFO: Why pay attention to Precigen right now?
Dr. Sabzevari: There are multiple reasons to pay attention to Precigen. First, our mission: The patient is at the center of everything we do and drives every aspect of our daily work. We believe what’s right for patients is right for the company and our stakeholders. Second, our technology and therapeutic platforms differentiate us from others. We have the technology platforms under our roof to enable cutting-edge science and new therapies to advance medicine with precision. We’ve talked about UltraCAR-T, which we were able to progress from discovery to the clinic in one year, but we also have other assets in development using other therapeutic platforms focused on other mechanisms to target cancers, such as vaccines, as well as therapies targeting autoimmune disorders and infectious diseases.
With the new Precigen, we are a dedicated gene and cell therapy company with unique technology and therapeutic platforms and a disciplined approach to fiscal responsibility and resource management that, we believe, will enable us to meet our goals. We also have a team of leaders and scientists that are performing at the highest level. They have dedicated their careers to helping patients and they are driving the Precigen portfolio forward with tremendous speed.
Precigen, Inc., Precision Medicine Cancer, Gene and Cell Therapy Companies, Nasdaq: PGEN, Dr. Helen Sabzevari, Precigen poised to disrupt gene and cell therapy industry with UltraCAR-T therapeutic platform, CEO Interviews 2020, Medical Companies, Biotech Company, precision medicine, multifunctional gene and cell therapy candidates, next generation of gene and cellular therapies, next gen gene therapy, next gen cell therapy, immuno-oncology, autoimmune disorders, and infectious diseases, non-viral and viral expression systems, genome, DNA, RNA and protein engineering, and a suite of precision bioengineering switch technologies controlling gene expression and regulation, on-target gene and cellular multifunctional therapies, Precigen, Inc., Press Releases, News










 Dr. Helen Sabzevari
Dr. Helen Sabzevari 